Discussion Forum : Alcohols Phenols And Ethers
Question -
At low temperature phenol reacts with Br2 in CS2to form
Answer: Option B
:
B
Was this answer helpful ?
:
B
In presence of non-polar solvent (CS2) the ionization of phenol is suppressed. The ring is slightly activated and hence mono substitution occurs with p-bromophenol as the major product.
On the other hand with water phenol forms 2, 4, 6-tribromo phenol. (CS2)
In aqueous solution phenol ionizes to give phenoxide ion. Due to the presence of negative charge of
oxygen the benzene ring is highly activated and hence trisubstituted product is obtained.
Was this answer helpful ?
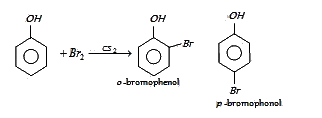
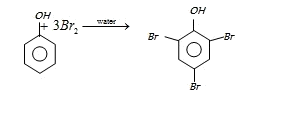
Submit Your Solution hear: