Discussion Forum : Chemical Kinetics
Question -
In the reaction, P + Q ⟶ R + S
the time taken for 75% reaction of P is twice the time taken for 50% reaction of P. The concentration of Q varies with reaction time as shown in the figure. The overall order of the reaction is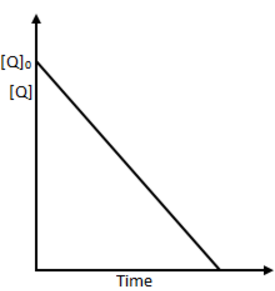
Answer: Option D
:
D
Therefore 1 is the right answer.
Was this answer helpful ?
:
D
Overall order of reaction can be decided by the data given t75﹪=2t50﹪
∴ It is a first order reaction with respect to P.
From graph [Q] is linearly decreasing with time, i.e., order of reaction w.r.t. Q is zero and the rate expression is r=[P]1[Q]0.
Therefore 1 is the right answer.
Was this answer helpful ?
Submit Your Solution hear: