Discussion Forum : P-block Group 18 - Noble Gases
Question -
The shape and geometry of XeF2 in vapor phase are respectively:
Answer: Option D
:
D
Let us look at the ground state valence electronic configuration of Xe and the first excited state:
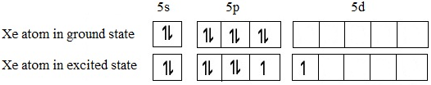
So there are 3 lone pairs of electrons and two bond pairs (each unpaired electron shown above will pair with exactly one electron from either of the Fluorine atoms). These orbitals may be assumed to be hybridized correspond to sp3d geometry.
The lone pairs occupy three ends of a symmetrical plane while the bond pairs will be axial or perpendicular to the plane containing the 3 electron pairs.
Shape is linear (due to perfect geometry) while the geometry is trigonal bipyramidal. What will be the F – Xe – F bond angle? Yes. It will will be 180 degrees.
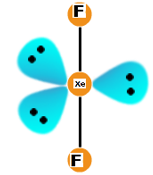
The geometry will be like:
Was this answer helpful ?
:
D
Let us look at the ground state valence electronic configuration of Xe and the first excited state:

So there are 3 lone pairs of electrons and two bond pairs (each unpaired electron shown above will pair with exactly one electron from either of the Fluorine atoms). These orbitals may be assumed to be hybridized correspond to sp3d geometry.
The lone pairs occupy three ends of a symmetrical plane while the bond pairs will be axial or perpendicular to the plane containing the 3 electron pairs.
Shape is linear (due to perfect geometry) while the geometry is trigonal bipyramidal. What will be the F – Xe – F bond angle? Yes. It will will be 180 degrees.
The geometry will be like:

Was this answer helpful ?
Submit Your Solution hear: