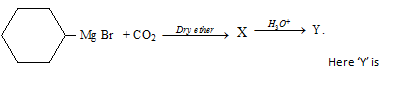Carboxylic Acids(11th And 12th > Chemistry ) Questions and Answers
Explanation:-
Answer: Option A. -> Dimer, Polymer:
A
In vapour 2 molecules are found together due to inter molecular hydrogen bond & in liquid many molecules are found together due to inter molecular hydrogen bond