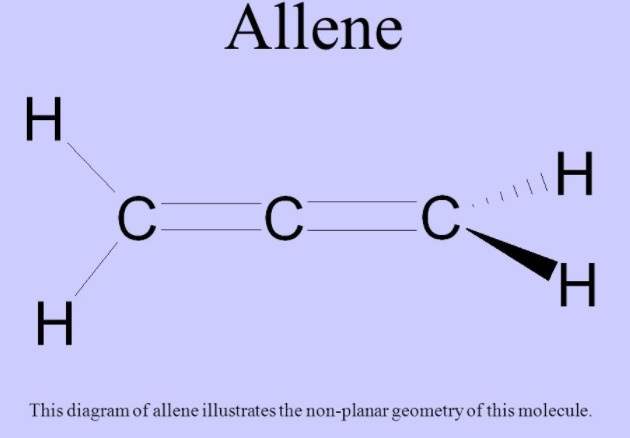
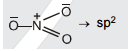
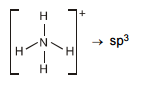
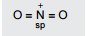Chemical Bonding(12th Grade > Chemistry ) Questions and Answers
Explanation:-
Answer: Option B. -> C2(CN)4:
B
C2(CN)4 molecule contains 9σ and 9 πbonds.
(CN)2−C=C−(CN)2
Each C bonded to N would have 2 pi and 1 sigma bond. So that is a total of 8 pi bonds. There is one pi bond between -C=C- in the middle.
The rest of the bonds are all sigma bonds - 1 each between C and N making a total of 4, 1 between the central carbon atoms, and 1 each between the central carbons and the C from CN, making a total of 9 sigma bonds.