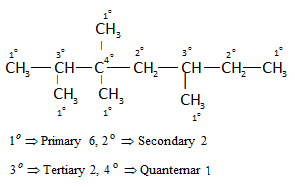
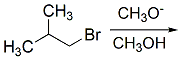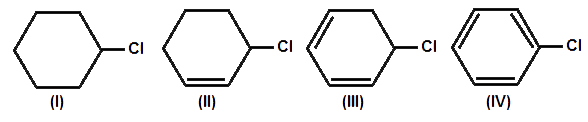Reaction Mechanism(12th Grade > Chemistry ) Questions and Answers
Explanation:-
Answer: Option B. -> The second reaction occurs faster than the first:
B
Polar aprotic solvents such as DMSO increase the rate of the SN2 reaction.
Question 6. A solution containing 0.2563 g of naphthalene (molecular mass = 128) in 50 g of carbon tetrachloride yields a boiling point elevation of 0.201∘C while a solution of 0.6216 g of an unknown solute in the same mass of the solvent gives a boiling point elevation of 0.647∘C. Find the molecular mass of unknown solute.
Explanation:-
Answer: Option A. -> 96.44:
A
We know that
Kb=ΔTb×W1×Mw21000×W2
First let’s calculate value of KbforCCl4.
For CCl4;
Kb=0.201×50×1281000×0.2563=5.019kkgmol−1
Now, for calculating mass of unknown solute, we can use the same value of Kb.
Mw2=1000×Kb×W2ΔTb×W1
1000×5.019×0.62160.647×50
=96.44